The Vast Benefits and Controversy Surrounding Stem Cell Research
Photo from Science Photo Library.
Aayush Iyengar, Pranav Karra, Geremiah Yang, & Jonathan Wang
I. Intro and Why We are Interested
Since the turn of the 21st century, stem cell research continues to be one of the most intriguing scientific research topics with an extremely high potential for human benefit. From the capability to slow cancer growth to the ability to heal heart defects, the benefits of continued stem cell research are numerous. However, the concept is not without its share of controversy; critics cite common issues including moral, religious, and practical issues regarding stem cell research. Regardless of the type of stem cell, from somatic to embryonic to induced pluripotent - all of which we will elaborate on within this paper - the progress of stem cell research has continuously been met with obstacles. Nevertheless, we strongly believe in the current and promising positives of stem cell research. The potential to eliminate countless genetic and terminal illnesses outweighs the negative moral problems, and the life-saving possibilities are what make stem cell research so revolutionary and fascinating not just to us but to scientists all over the world.
II. Background Facts
Before diving deeper into the vast benefits of stem cell research, there are a few different categories of stem cells that need to be understood. Known as the body’s raw material, stem cells are the origins of over 200 types of cells. With the ability to differentiate, or specialize in a specific function, stem cells are responsible for the development, growth, and regeneration of the body. Under the correct conditions, stem cells undergo mitosis, producing identical daughter cells. These daughter cells, through multiple generations of cell division, then go on to specialize into specific cells with specific functions.
This intrinsic ability of differentiation is dictated through a series of signal transduction pathways and gene expression. Stem cells contain a protein that is responsible for the regulation of genes that promote differentiation into certain cell types. According to a new study published in Molecular Cell, “OCT4 is a stem cell-specific transcription factor—a protein that regulates gene activity—that maintains stem cells’ ability to differentiate into any tissue in the body. OCT4 works by sitting on DNA and recruiting factors that either help initiate or repress the reading of specific genes, in order to become specialized” (Moore, 2016). In short, the stem cells contain a protein called OCT4, which regulates gene expression in order to differentiate into a specific type of somatic cell.
To achieve further specialization, the OCT4 protein collaborates with other transcription factors that are activated by certain external signals through signal transduction pathways. “The new study shows that, in certain genes, OCT4 also collaborates with transcription factors that are activated by external signals, such as the retinoic acid (vitamin A) receptor (RAR) and beta-catenin, to turn on their respective genes” (Moore, 16). Many transcription factors are involved in the process of differentiation. These factors are activated by external signals from vitamins, and stimulate the process of growth through the differentiation of one’s stem cells.
However, not all stem cells are the same, and they are classified into 3 general types: Adult, Induced pluripotent (IPC), and embryonic stem cells. Adult stem cells are found in small numbers in most adult tissues, such as bone marrow and fat. Just like any stem cell, they have the ability to specialize into different cells for regenerative purposes. However, adult stem cells are already developed and partially differentiated, which limits their range of differentiation compared to the other types of stem cells. For example, adult stem cells found in the skin are only able to differentiate into specific cells that are directly associated with the skin. Although scientists have created successful stem cells that are capable of more diverse differentiation ability, the research is still in the developing stages and is not a viable substitution for embryonic stem cells.
Embryonic stem cells are totipotent cells, meaning they have the ability to specialize into any type of somatic cell. These stem cells come from embryos that are 3 to 5 days old. At this stage, an embryo is called a blastocyst and has about 150 cells. This universal versatility enables embryonic stem cells to regenerate, repair diseased tissue and organs, and even create a complete human body. This characteristic alone is why embryonic cells are considered crucial in the future of medical technology and treatment.
The last type of stem cells are Induced pluripotent cells (IPCs), which are cells derived from normal skin or blood cells that have been reprogrammed back into an embryonic-like pluripotent state with the purpose of developing into an unlimited source of any type of human cell needed for therapeutic purposes.
III. Medical and Moral Benefits
In terms of biomedical research, adult stem cells are the only types of cells that have effectively treated human patients. Over 50,000 people are treated with adult stem cells each year. There are published successes in treating basic cancers, spinal cord injuries, heart damage, multiple sclerosis, sickle cell anemia, and many other ailments. Doctors at Cedars-Sinai Hospital in Los Angeles have shown that adult stem cells can regrow damaged heart muscle and reduce scars; Doctors at Yale University and the Texas Heart Institute have separately demonstrated success in using adult stem cells to fix the heart; Italian doctor Paolo Macchiarini even used adult stem cells to grow a replacement windpipe; French scientists successfully used adult stem cells to create and grow blood transfusions, a treatment that is extremely beneficial for people with very rare blood types whose donors aren’t widely available. Additionally, contrary to popular beliefs, acquiring these stem cells from the patient isn’t harmful and has little to no side effects. Even in the fledgling stages, the research is extremely promising and beneficial (Prentice 32). However, due to the limited ability of the adult stem cells, doctors are restricted in the types of treatments they can administer using solely adult stem cells. Therefore, the use of embryonic stem cells is heavily sought out.
Embryonic stem cells have the potential to cure any type of disease or condition that requires the regeneration of cells and tissues. For example, most terminal brain diseases, such as Alzheimer’s and Parkinson's, deal with the deterioration of brain cells. As such, scientists can use embryonic stem cells to replace and regenerate the damaged areas in the brain. This research can treat diabetes, injuries from burns, paralysis (spinal cord injuries), and more. Furthermore, embryonic stem cells can replace cells damaged by chemotherapy, disease, or cancer. Even now, research is being conducted on the viability of embryonic stem cells to differentiate into certain types of immune system cells to fight against many types of cancer and blood-related diseases, such as leukemia, lymphoma, neuroblastoma, and multiple myeloma (Forman 20).
Embryonic stem cells not only have the potential for current medical treatment but also for new forms of research and testing for future medical advancement. Before testing investigational drugs (laboratory-approved drugs) in people, researchers can use stem cells to test for safety and quality. They use stem cells to grow organs, which then can be used to simulate the effects of drugs without risking human lives. For the testing of new drugs to be accurate, the cells must be programmed to acquire the properties of cells that would normally be targeted by the drug. For instance, nerve cells can be generated to test a new treatment for nerve disease that would show whether the new treatment had any effect on the disease and whether the nerve cells were harmed. We believe that testing on actual human cells and organs is more representative of the drug's effects compared to laboratory testing on rats or rabbits. Thus, by improving the accuracy, efficacy, reliability, and safety of drug testing, advancement in stem cell research can be considered the next leap forward for pharmaceuticals (Forman 24).
IV. Ethical and Financial Negatives
To get a full perspective of a topic as controversial as stem cell research, the views opposed to stem cell research must be considered as well. The biggest moral issue is the method of harvesting human embryonic stem cells (hESCs). In order to harvest hESCs, it is necessary to destroy the five-day-old preimplantation embryo. Opponents of hESC research argue that because the embryo has the capability of developing into a human being, it has a significant moral standing; therefore, its destruction is unethical and is essentially equal to the killing of a human being.
Additionally, religion can explain why some are against this research. Religions such as Catholicism and Hinduism respect and value all stages of life and are strongly against the deliberate destruction of human lives. In these religions, it is believed that the loss of life of an embryo is not worth the development of research and technology. Additionally there is controversy surrounding women donating their eggs for stem cell research. Opponents argue that even if a female’s donated egg isn’t fertilized yet, it has the potential to become human, and is therefore considered unethical, according to their religion.
While the disadvantages of conventional embryonic stem cells make IPCs seem like the better solution, it is important to address that the viability of using IPCs is extremely low. According to an article by Dr. Surat P. on News-Medical.net, the successful reprogramming rate of somatic cells to IPCs is less than 0.02% and possesses a prevalent tendency of improper differentiation, deeming them currently unviable for medical application.
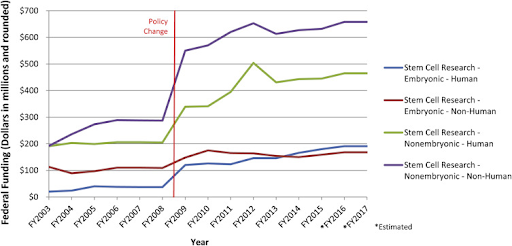
The biggest issue towards the acceptance of stem cell research in society currently is the exuberant cost of the research due to the technology required. According to a published Californian article, “With its original Proposition 71 funds of $3 billion running out, beneficiaries of the California Institute for Regenerative Medicine (CIRM) are looking to draw out another $5.5 billion in taxpayer-funded bonds for stem-cell research that after 15 years has produced close to no results'' (Moorlach, 2019). Taxpayers in California are understandably displeased with the large amount of their money that was spent on the research, with little results to show. Therefore, this has led many to wonder if stem cell research is possible, let alone practical. Critics also argue that these large sums of money could be spent on other medical issues that yield tangible results. In other words, stem cell research opponents consider the cost of results too much for too little results.
V. History of Legislation
Due to the nature of stem cell research, there have been many changes to the laws and regulations. The first major regulation put in place occurred around 1996 in the Dickey-Wicker amendment. The amendment prohibited government funding for any work that would harm or destroy a human embryo. At the time, researchers circumnavigated issues with the law by avoiding the destruction of the embryo; however, resistance against embryonic stem cell research continued.
On August 9, 2001, U.S. President George W. Bush introduced a ban on federal funding for research on newly created human embryonic stem cell lines. The ban essentially promoted research on already destroyed embryos and prohibited government funding for research on alive human embryonic stem cells. However, In 2009, President Obama issued an executive order that overturned President Bush’s limitations on embryonic stem cell research.
In recent years, the Trump administration issued a stop to fetal-tissue research by government scientists and introduced a requirement that any grant applications involving such material must undergo an additional ethics review. As a result, US researchers are required to have their research approved by a research oversight committee before they receive any federal funding. This law resulted in many researchers seeking private funding instead of federal funding and reduced the already slow progress of stem cell research.
Although there is a variance in state-laws, there are some common laws that stem cell researchers follow. For one, stem cell researchers must inform and receive consent from donors before any work is started. Researchers must also be guided by an ethical guideline called the 14-day rule, which limits embryo research to within the two weeks after fertilization. All in all, with such a new and controversial topic, laws will continue to be put into place to regulate and control the developing research and make it as ethical as possible
VI. Conclusion and Our Stance
As shown by the various arguments mentioned before, the debate about stem cell research is a complex, moral, political, and ethical problem. However, there are many benefits to stem cell research from the ability to save lives and the potential for future cures and treatments for many terminal diseases. Consequently, we ultimately believe that the ends justify the means for medical development.
Since every member in our group is passionate about medical advancement, we share a perspective that is more focused on the scientific possibilities and less emotionally attached to the necessary procedure. Our perspective leads us to believe that embryos have a lesser moral status than humans and we can easily dissociate ourselves from others’ belief that life starts at conception. Not to mention, we feel that if there is a possible solution that can lead to saving thousands of lives, we are obligated to research the subject. Overall, we concluded that the moral status of an embryo is unclear and cannot be equated to that of adult life. As long as the research is done within the necessary protocols, we believe that human embryonic stem cell research is morally justified.
Our stance is closely aligned with that of Congresswoman Diana DeGette. DeGette stated that “there are fewer than 200 snowflake babies, and 400,000 frozen embryos are slated to be thrown away” (Forman 15). Instead of wasting these embryos from VITRO facilities, she argues that it would be more reasonable for researchers to use stem cells from frozen embryos to find cures. The combination of current embryonic stem cell resources along with the aforementioned potential benefits of such research greatly influence our belief that stem cell research is beneficial and must be continued. Above all else, we passionately believe that the utilization of embryos is a necessary cost that can unlock the ability to save countless amounts of countless lives. Although the past legal environment has been restrictive on embryonic stem cell research, we believe that as time goes on, the government will lift regulations surrounding stem cell research, allowing for rapid progress. While choosing a side on this debate may seem impossible, we believe that instead of looking at this topic in the scope of a small population of embryos, one should truly look at the endless number of generations this research could benefit.
Works Cited
“Answers to Your Questions about Stem Cell Research.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 19 Mar. 2022, www.mayoclinic.org/tests-procedures/bone-marrow-transplant/in-depth/stem-cells/art-20048117#:~:text=Stem cells are the body's raw materials — cells from which,more cells called daughter cells.
Article, and Dana G. Smith. “Stem Cell Supply and Demand.” Home, 20 June 2016, gladstone.org/news/stem-cell-supply-and-demand#:~:text=The process is tedious, time,develop into the appropriate cells.
Charitos, Ioannis Alexandros, et al. “Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues.” Stem Cells International, Hindawi, 30 Apr. 2021, www.hindawi.com/journals/sci/2021/9978837/.
Commission, National Bioethics Advisory. “IUPUI.” Ethical Issues in Human Stem Cell Research, National Bioethics Advisory Commission, Rockville, MD, 1 Sept. 1999, scholarworks.iupui.edu/handle/1805/23.
Forman, Lillian. Stem Cell Research. ABDO Pub., 2008.
Garcia, Tahra Johnson; Alise. Embryonic and Fetal Research Laws, www.ncsl.org/research/health/embryonic-and-fetal-research-laws.aspx.
Hunnicutt, Susan. Embryonic and Adult Stem Cells. Greenhaven Press, a Part of Gale, Cengage Learning, 2013.
John P. Cunha, DO. “Is Stem Cell Research Illegal in The United States?” EMedicineHealth, EMedicineHealth, 2 Dec. 2020, www.emedicinehealth.com/is_stem_cell_research_illegal_in_the_united_states/article_em.htm.
King, Nancy MP, and Jacob Perrin. “Ethical Issues in Stem Cell Research and Therapy - Stem Cell Research & Therapy.” SpringerLink, BioMed Central, 7 July 2014, link.springer.com/article/10.1186/scrt474.
Lo, Bernard, and Lindsay Parham. “Ethical Issues in Stem Cell Research.” OUP Academic, Oxford University Press, 1 May 2009, academic.oup.com/edrv/article/30/3/204/2354990?login=true.
Moorlach, John M. W. “Commentary: Why Stem-Cell Research Funding Proposal Is a Waste of Taxpayer Money.” Tribune, San Diego Union-Tribune, 25 Sept. 2019, www.sandiegouniontribune.com/opinion/story/2019-09-25/commentary-why-stem-cell-research-funding-proposal-is-a-waste-of-taxpayer-money.
Murugan, Varnee. “Embryonic Stem Cell Research: a Decade of Debate from Bush to Obama.” The Yale Journal of Biology and Medicine, YJBM, Sept. 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2744932/#:~:text=On August 9, 2001, U.S.,still be eligible for funding.
“News.” Breakthrough in Understanding How Stem Cells Become Specialized | Sanford Burnham Prebys, 4 Aug. 2016, www.sbpdiscovery.org/news/beaker-blog/breakthrough-understanding-how-stem-cells-become-specialized.
P, Dr. Surat. “Induced Pluripotent Stem (IPS) Cells: Discovery, Advantages and CRISPR Cas9 Gene Editing.” News, 17 July 2019, www.news-medical.net/life-sciences/Induced-Pluripotent-Stem-(iPS)-Cells-Discovery-Advantages-and-CRISPR-Cas9-Gene-Editing.aspx#:~:text=Muhammad Khan | TEDxBrentwoodCollegeSchool-,Disadvantages,trigger cancer-causing gene expression.
Staff, Ask A Scientist, et al. “What Are Stem Cells?” Ask a Scientist, 1 Nov. 2019, www.thermofisher.com/blog/ask-a-scientist/what-are-stem-cells/.
Subbaraman, Nidhi. “Research on Embryo-like Structures Struggles to Win US Government Funding.” Nature News, Nature Publishing Group, 17 Jan. 2020, www.nature.com/articles/d41586-020-00127-z.
Thomas, Isabel. What Is the Controversy over Stem Cell Research? Capstone Global Library Ltd, 2013.
“The Truth About the Dickey-Wicker Amendment.” SBA Pro-Life America, 25 Sept. 2015, sbaprolife.org/suzy-b-blog/truth-about-dickey-wicker-amendment.
Zakrzewski, Wojciech, et al. “Stem Cells: Past, Present, and Future - Stem Cell Research & Therapy.” BioMed Central, BioMed Central, 26 Feb. 2019, stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1165-5#:~:text=Human embryonic stem cells (hESCs,the adult organism [2].
“Default - Stanford Children's Health.” Stanford Children's Health - Lucile Packard Children's Hospital Stanford, www.stanfordchildrens.org/en/topic/default?id=what-are-stem-cells-160-38.